一、 What is a fluorescent label?
The compounds on which the fluorescent labeling depends are called fluorescent substances.Fluorescent substances refer to compounds with the chemical structure of conjugated double bond system, which can be excited to an excited state when irradiated by ultraviolet light or blue-violet light, and fluorescent when restored to the ground state from the excited state.Fluorescent labeling technology refers to the use of fluorescent material covalently bound or physically adsorbed on a group of the molecule to be studied, using its fluorescence characteristics to provide information of the subject of study.The advantages of fluorescent labeling, such as no radiation pollution and easy operation, make the application of fluorescent labeling in many research fields increasingly widespread.
二、 Role of fluorescent labeling
Fluorescent markers have also been widely used in protein function research, drug screening and other fields.Fluorescent-labeled polypeptides have been used to detect the activity of target proteins, and their developed high-throughput activity screening methods have been applied to drug screening and drug development of target proteins for disease treatment (e.g., various kinases, phosphatases, peptidases, etc.).Therefore, the fluorescence modification of polypeptides is also an important content in the field of polypeptide synthesis.
三、 Fluorescent labeled sites
Fluorescent markers can be directly linked to the N-terminus of polypeptides (e.g., fluorescein isothiocyanate, usually inserted directly into an aminoacetic acid or a beta-alanine at the N-terminus of polypeptides and fluorescein isothiocyanate).Or they are linked at the C-terminal side chain with lysine (or cysteine), or at other positions that can be linked.
四、 Examples of fluorescent labeling
4.1 FITC修饰
Fluorescein isothiocyanate (FITC) has relatively high activity. Generally, it is easier to introduce this fluorescent group in the solid-phase synthesis process than other fluoresceins, and no activating reagent is needed in the reaction process.
FITC-modified polypeptides usually have two main forms:
(1)FITC is accessed at the N-terminus of the entire peptide chain and one molecule of Acp (6-aminocaproic acid), also known as an alkyl spacer, is accessed before FITC.In the reaction, FITC reacts with the bare -NH2 on the peptide chain, and the access of Acp provides a straight-chain space of six carbons, which greatly reduces the steric hindrance of the reaction, improves the reaction efficiency and reduces the difficulty of the reaction.Secondly, FITC also reacts with -SH, side chain-NH2 in the polypeptide structure, and the addition of Acp also reduces the possibility of this side reaction.In addition, when polypeptides are cleaved under acidic conditions, the polypeptides that access FITC at the N-terminus need to undergo cyclization to form fluorescein, a process usually accompanied by the removal of the last amino acid, which is avoided by the introduction of the alkyl spacer Acp.
(2)FITC is linked to one of the Lys side chains in the whole peptide, and the Lys side chain is a four-carbon linear alkyl group terminated with -NH2, which directly plays a role in reducing the steric hindrance.This modification method enables flexible FITC modification anywhere in the entire peptide, not just at the end.
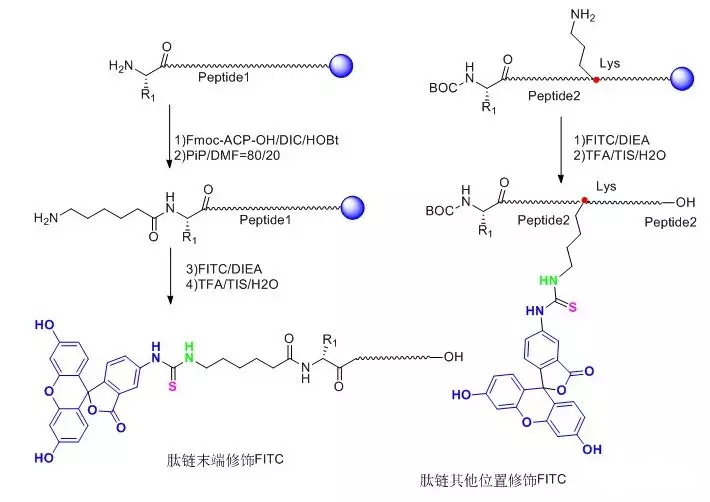
Graphical FITC Modification
4.2 AMC modification
7-Amino-4-methylcoumarin (AMC) is a widely used fluorescent labeling reagent, for example, trace determination of enzymes, identification of enzymes, preparation of laser dyes and other uses. In addition, the C-terminal ubiquitin molecule modified with coumarin is also an important probe to study the ubiquitination process of proteins.
Unlike other fluorescent dyes, AMC-modified polypeptide molecules are carried out from the C-terminus: (1) AMC reacts with the first amino acid at the C-terminus of the peptide chain;(2) Solid-phase synthesis of the whole peptide chain (starting from the second amino acid), and retaining the side chain protecting group and the last amino protecting group of the whole peptide chain; (3) liquid-phase condensation of AA-AMC with the fully protected peptide chain; (4) excision of the protecting group to complete the modification of the peptide chain.
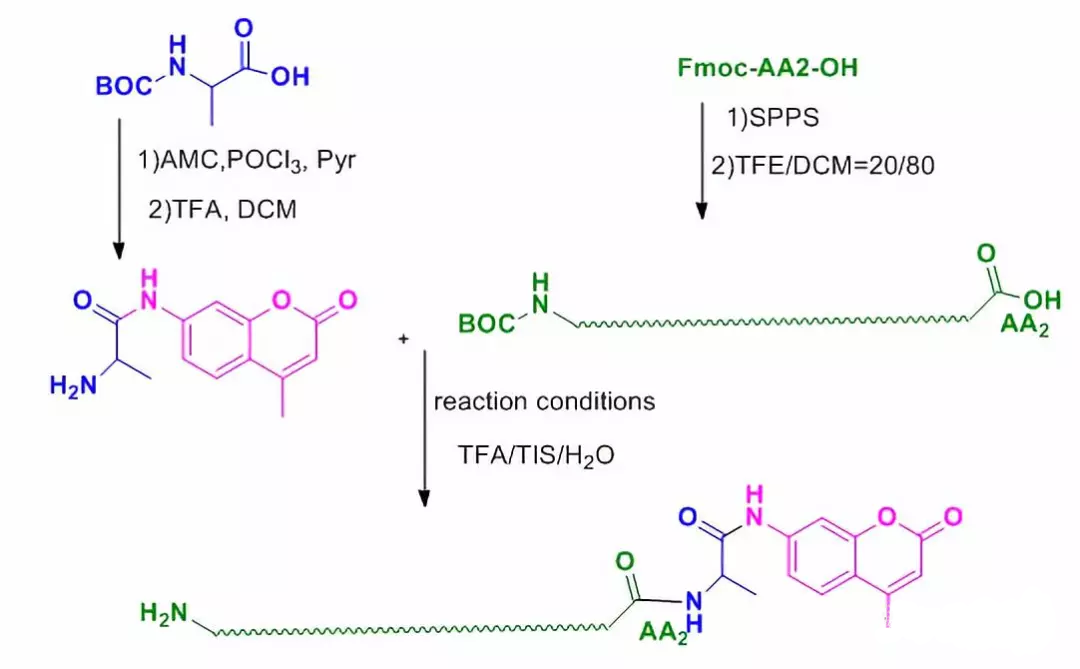
Graphical AMC Modification
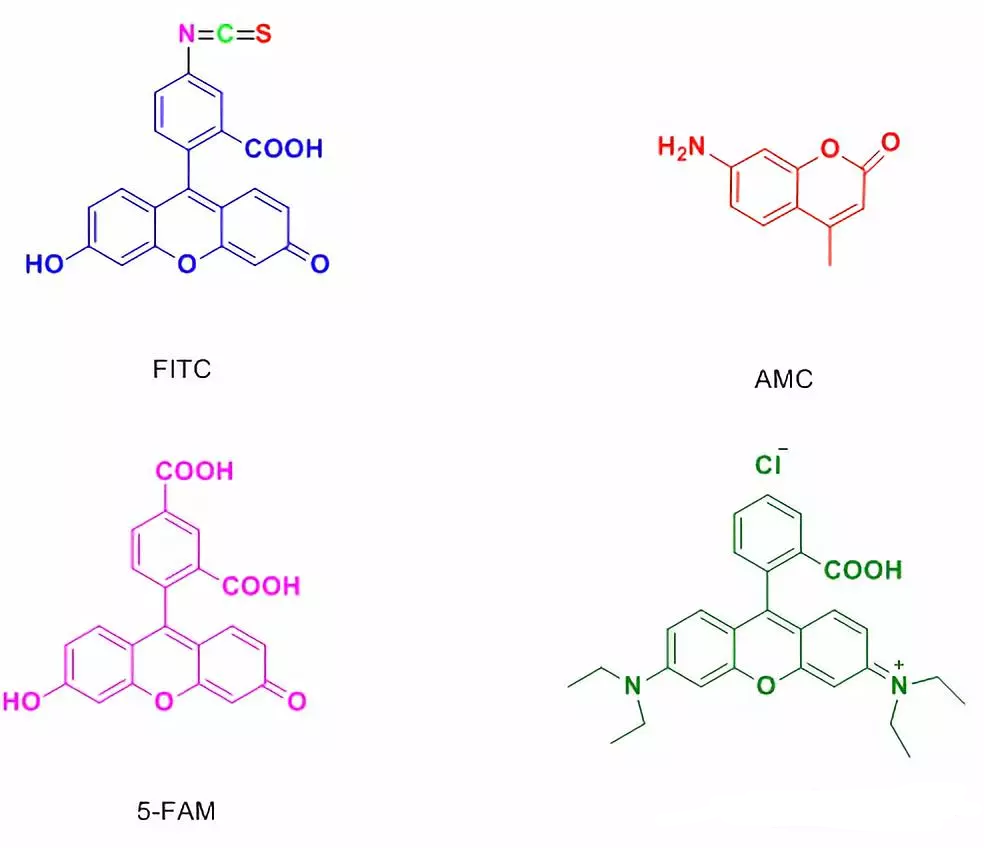
4.3 5-FAM修饰
Usually used in laser confocal microscopy and flow cytometry.
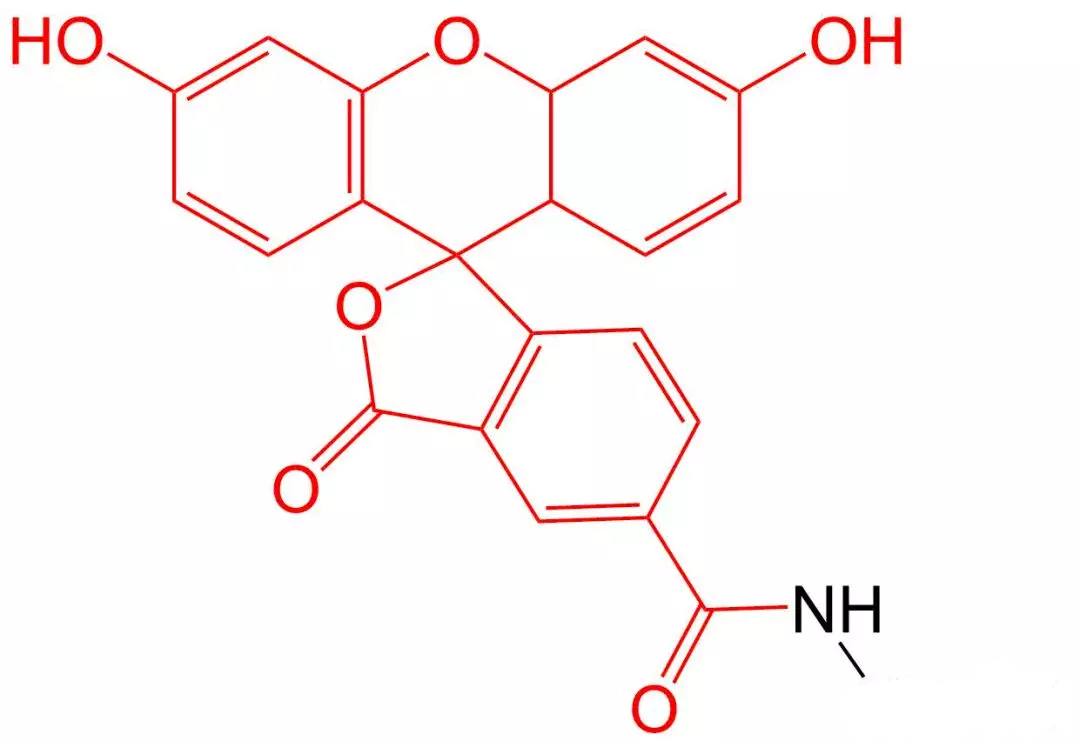
4.4 Rhodamine B (Rhodamine B) Modification
Rhodamine B is one of many rhodamine dyes used for fluorescence determination.
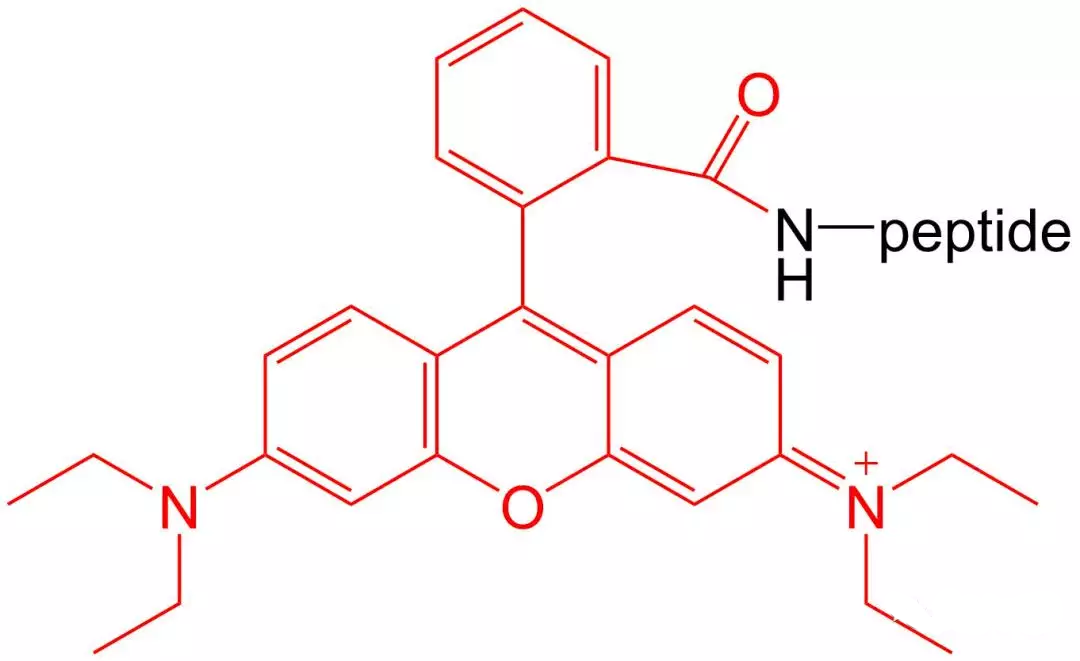
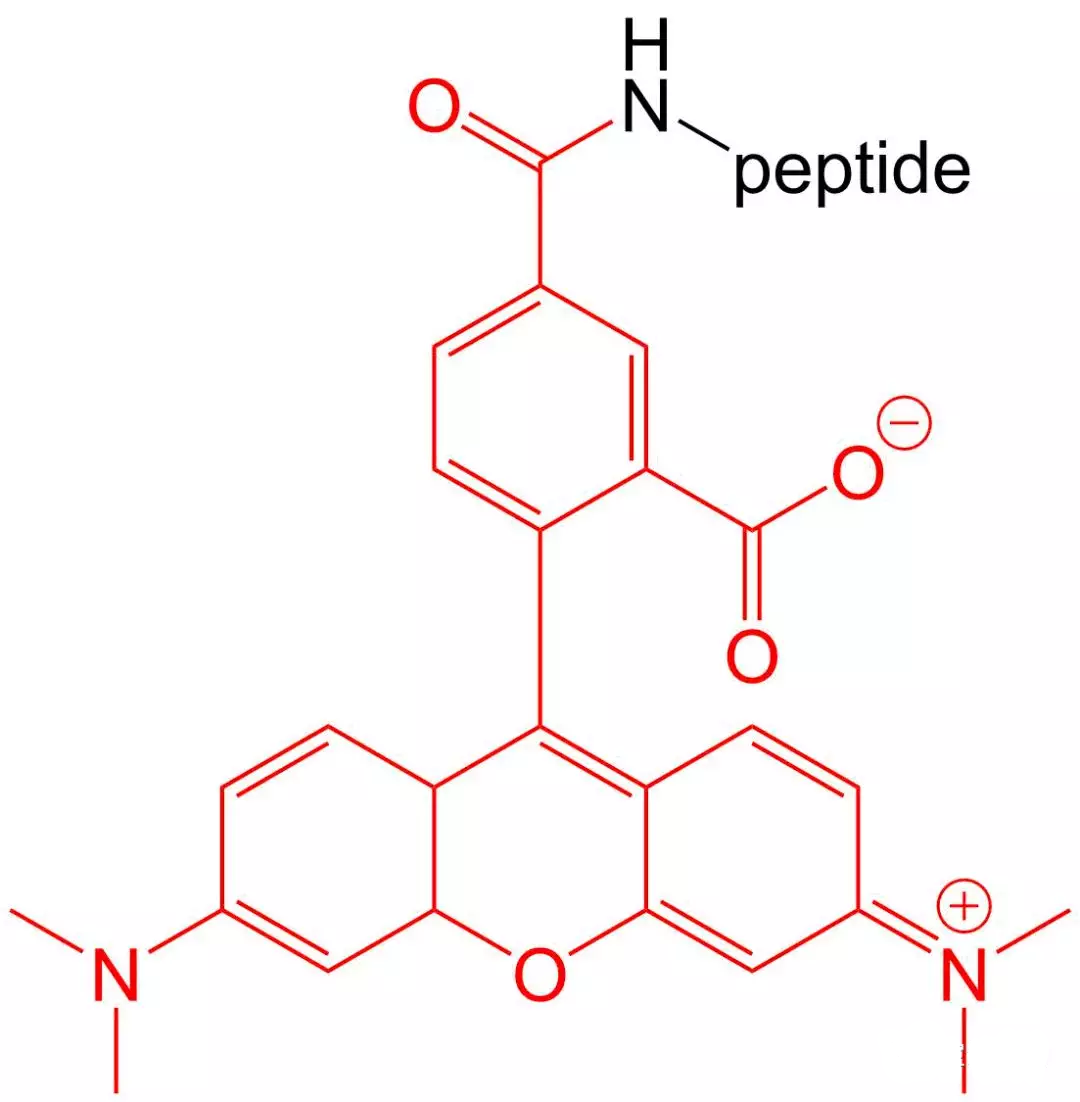
4.5 5- TAMRA Modification
5-TAMRA is one of the most popular orange fluorophores used to label peptides and proteins.
4.6 Cy 3 and Cy 5 modifications
Cy3 and Cy5 are dyes with high extinction coefficients.Therefore, they are particularly suitable for intracellular sensitive polypeptide localization experiments.Their disadvantage is that they are very unstable in the environment of polypeptide synthesis, so the amount of synthesis is relatively low.
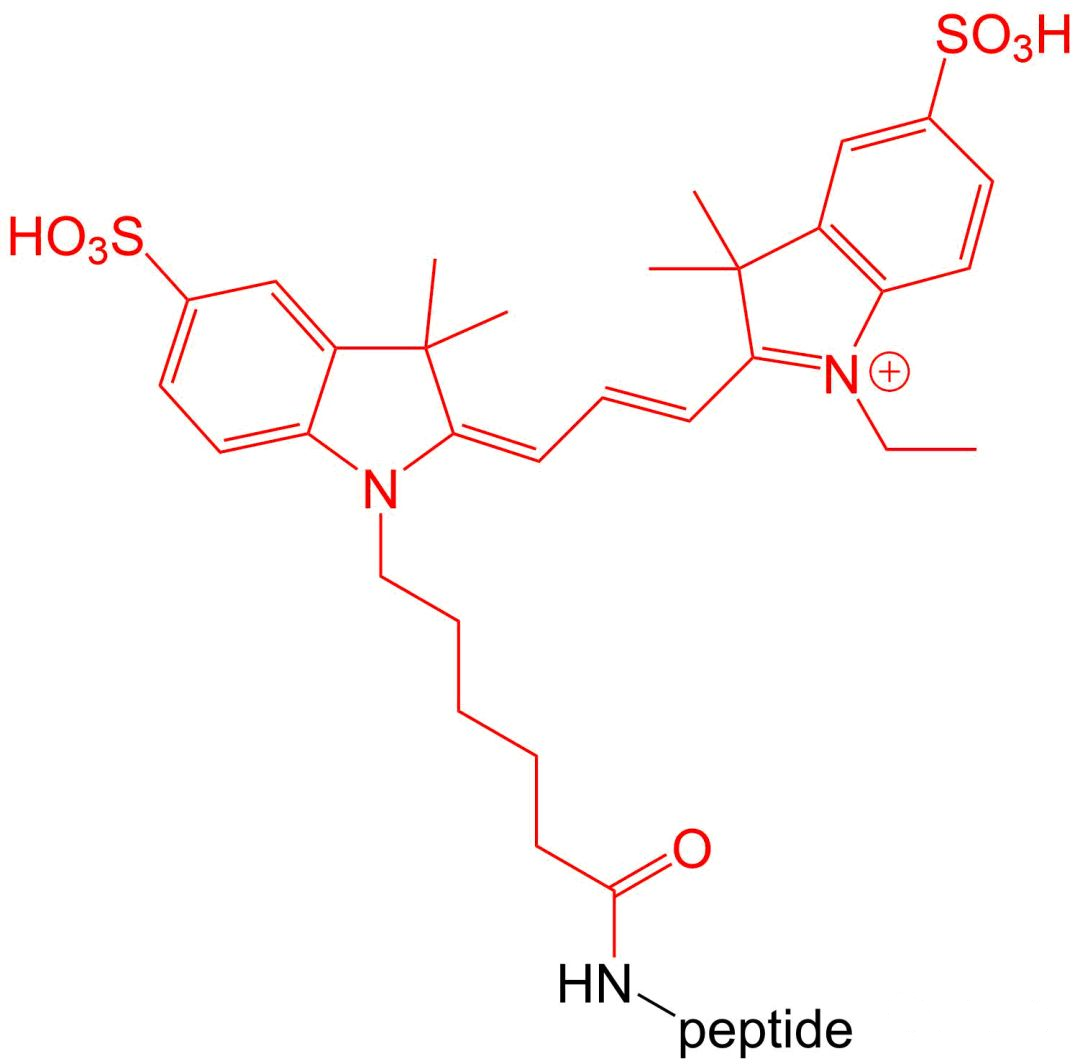
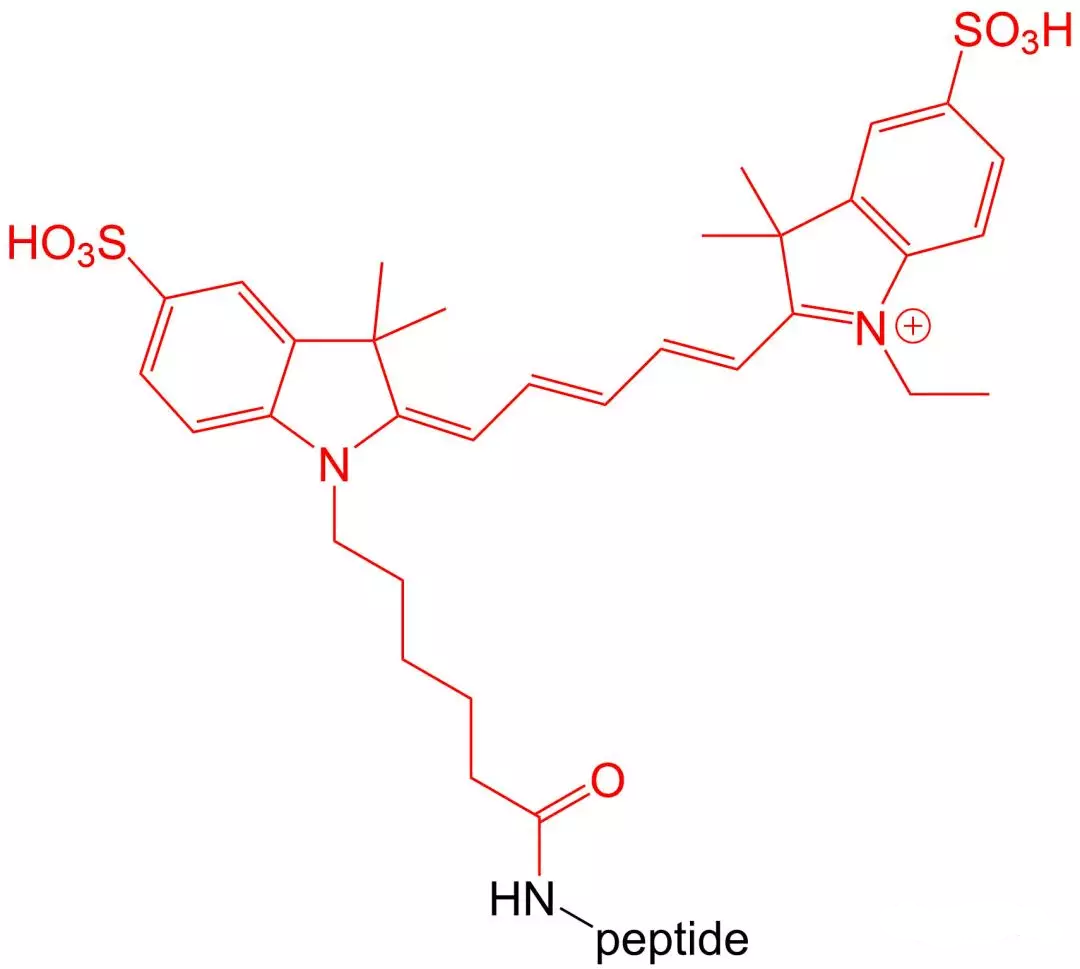
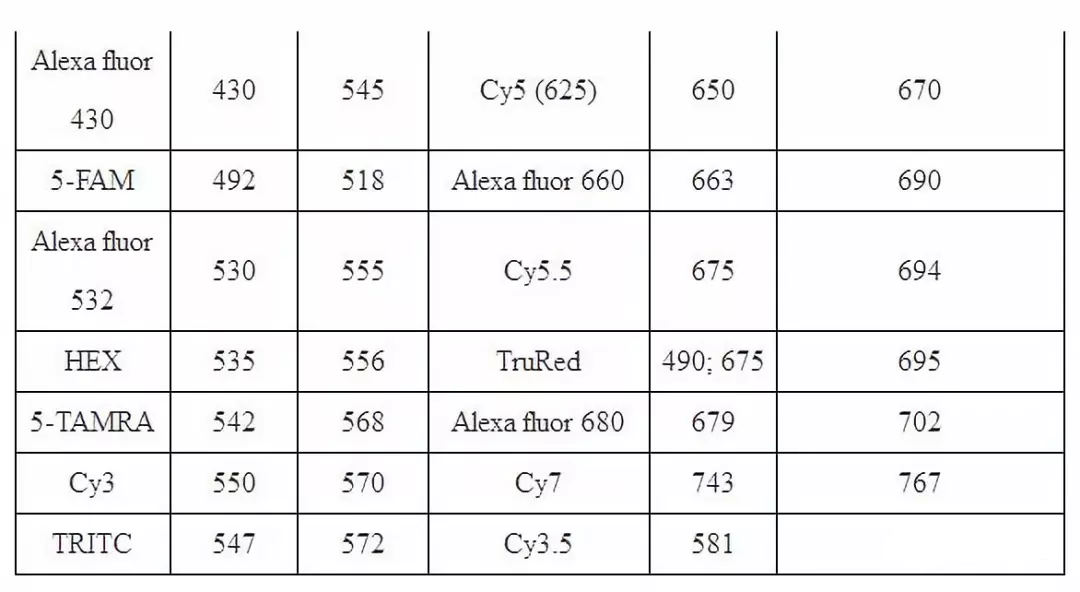
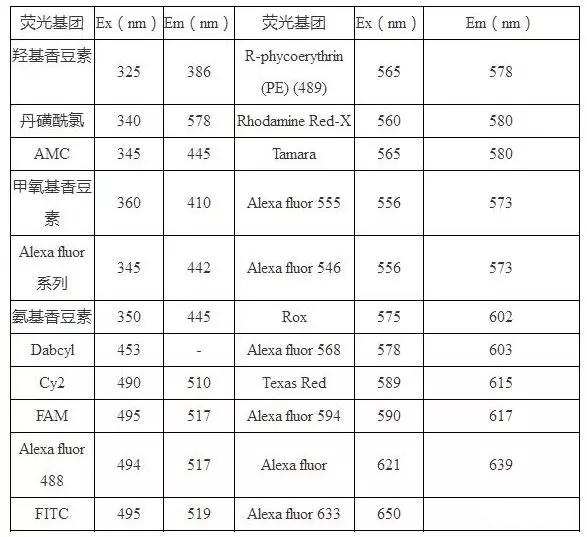
五、 Wavelength and excitation wavelength of common fluorescent substances