Liquid phase peptide synthesis(solution phase synthesis) principle
Based on adding a single n-α protected amino acid to the growing amino acid repeatedly, the synthesis proceeds step by step, usually starting from the C-terminal amino acid of the synthesis chain, and then the connection of the single amino acid is realized by using DCC, mixed carboxyanhydrides or N-carboxyanhydrides.Carbodiimide The method includes using DCC as linker to connect n - and C-protected amino acids.It is important that the linker can promote the shrinkage between the carbon group of N-protected amino acid and the free amino group of C-protected amino acid to form peptide chain and produce by-products at the same time. However, this method is affected by the side effects of racemization or the formation of 5 (4h) - oxaylones and n-acylurea in the presence of strong bases.Fortunately, these side effects can be minimized, if not completely eliminated.In addition, the method can also be used to synthesize the active ester derivatives of N-protected amino acids.The active ester produced in turn will spontaneously react with any other C-protected amino acid or peptide to form a new peptide.
Liquid phase peptide synthesis is still widely used now. It has the obvious advantages of large scale and low cost in the synthesis of short peptides and peptide fragments. Moreover, due to the reaction in homogeneous phase, the reaction conditions that can be selected are more abundant, such as catalytic hydrogenation, alkaline hydrolysis and other conditions, which can be used in solid phase, but due to the low reaction efficiency and side effects Reaction and other reasons cannot be applied.BOC and Z are two main reaction strategies in liquid peptide synthesis.
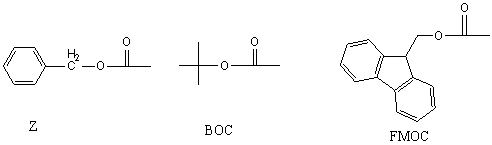
Schematic diagram of liquid phase synthesis Glu TRP
Condensation reagents mainly include:Carbodiimide type, onium salt type(Uronium)
Carbodiimide type
Mainly including: DCC, DIC, edc.hcl, etc.DCC is used in the reaction, because the DCU generated in the reaction has little solubility in DMF and produces white precipitate, so it is generally not used in the solid-phase synthesis, but because of its low price, it can be removed by filtration in the liquid-phase synthesis, which is still widely used.Because of its water solubility, edc.hcl is widely used in the connection of peptides and proteins, and it is also quite successful.However, one of the biggest disadvantages of this type of condensation reagent is that if it is used alone, there will be more side reactions. However, the research shows that the side reactions can be controlled in a very low range if HOBt, hoat and other reagents are added in the activation process.
uronium
Because of its high reaction activity and high speed, onium salt type condensation reagent is widely used now, mainly including HBTU, tbtu, Hatu, pybop, etc.During the use of the reagent, it is necessary to add organic bases, such as diisopropyl ethylamine (diea) and N-Methylmorpholine (NMM). Only after the reagent is added can amino acids be activated.
Characteristics of liquid phase reaction:
Most classical chemical reactions are carried out in solution.So:
(1)In solution phase synthesis, all previous organic synthesis methods can be used without any limitation;
(2)The reactants are uniformly mixed and move rapidly to increase the reaction opportunity;
(3)In the case of a heating reaction, the heat energy is uniformly transferred by the dispersion of molecules in the solution;
(4)A large number of reactions can be realized by controlling the size of the reactor and the number of reactants;
(5)The reactive compounds can be purified and analyzed at each step.
Disadvantages:
(1)After the completion of the reaction, the required compounds and by-products are in the reaction mixture together, which requires the separation step in solution chemistry.
(2)If excess reagents are used to achieve high yields, the reagents need to be purified.
(3)If the starting material or by-product (or the required compound) is volatile or precipitated, it is much easier. However, if these do not occur, a more difficult post-processing work - extraction or chromatography is required. Therefore, post-processing usually requires more time and energy than reaction process.
(4)Because of the complexity of purification procedure, it is very difficult to realize automatic solution phase synthesis.