Description 1:
Protein and polypeptide drugs have the advantages of specific site of action and clear efficacy. In recent years, the research and development of protein and polypeptide drugs have become a hot spot in the field of biomedicine.Disulfide bonds play an important role in maintaining the spatial stereostructure of polypeptides and proteins and the resulting biological activity.The disulfide bond is the S-S covalent bond formed by oxidation of the sulfhydryl group (-SH) of Cys at two different sites in a protein or a polypeptide molecule.A disulfide bond formed between amino acids at different positions in a peptide chain can fold the peptide chain into a specific spatial structure.
、
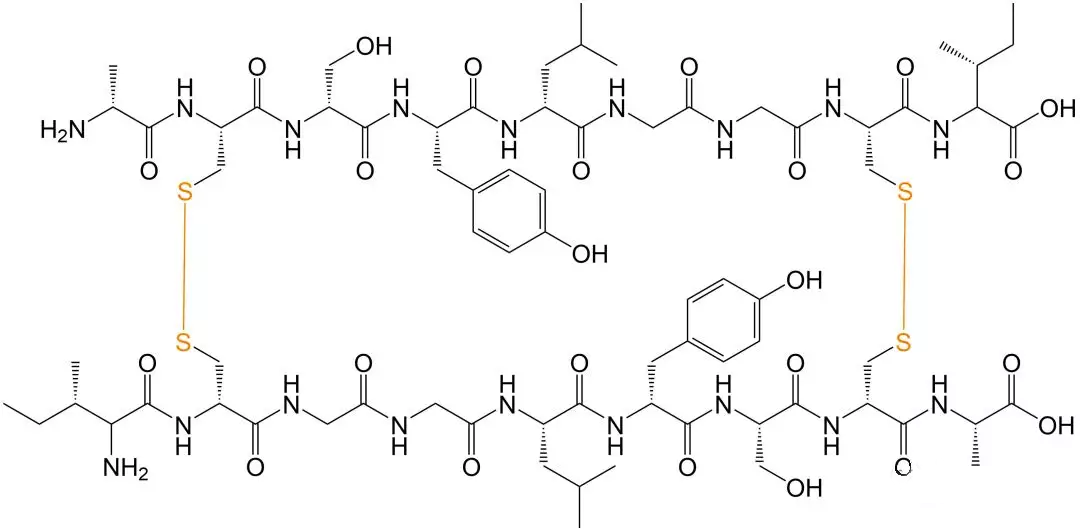
Schematic Diagram of Intermolecular Disulfide-bonded Cyclic Peptides
Polypeptide molecules usually have large molecular weight and complex spatial structure. When disulfide bonds are formed in the structure, two cysteines are required to be close in space distance.In addition, the sulfhydryl group in the reduced state in the polypeptide structure is active and prone to other side reactions, and other side chains on the peptide chain may also undergo a series of modifications. Therefore, the oxidant and oxidation conditions selected for the modification of the peptide chain are the key factors of the reaction, and the reaction mechanism is also complex, which may be either free radical reaction or ionic reaction.
Pyronium salt condensation reagent has high reaction activity and high speed, and is widely used nowadays, mainly including: HBTU, TBTU, HATU, PyBOP, etc.Organic bases such as diisopropylethylamine (DIEA) and N-methylmorpholine (NMM) need to be added during the use of this reagent to activate amino acids.
In the modification of disulfide bonds of polypeptides, the synthesis of a pair of disulfide bonds within or between molecules is usually relatively easy. There are many choices of reaction conditions, such as air oxidation, DMSO oxidation and other mild oxidation processes, and also intense reaction conditions such as H2O2, I2, mercury salts can be used. The reaction products are also relatively easy to purify and separate, resulting in higher purity and yield.
The formation of disulfide bonds by air oxidation is the most classical method in polypeptide synthesis, and good results have been obtained in early studies.The polypeptide with sulfhydryl group in the reduced state is usually dissolved in water by air oxidation, and reacted for more than 24 hours under near neutral or weak alkaline conditions (PH value 6.5-10).In order to reduce the possibility of disulfide bond formation between molecules, this method usually needs to be carried out under low concentration conditions.
Iodine oxidation method is also widely used in polypeptide synthesis. Generally, polypeptides are dissolved in 25% methanol aqueous solution or 30% acetic acid aqueous solution, and 10-15 mol/L of iodine is added drop by drop to oxidize, and the reaction lasts for 15-40 min.When the peptide chain contains residues of Tyr, Trp, Met and His which are sensitive to iodine, the oxidation conditions should be controlled more precisely. After oxidation, vitamin C or sodium thiosulfate are added immediately to remove excess iodine.
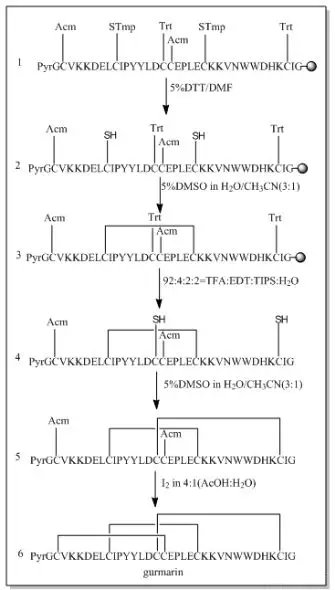
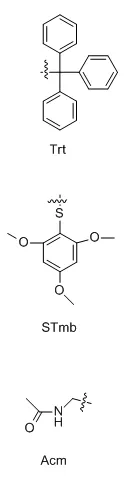
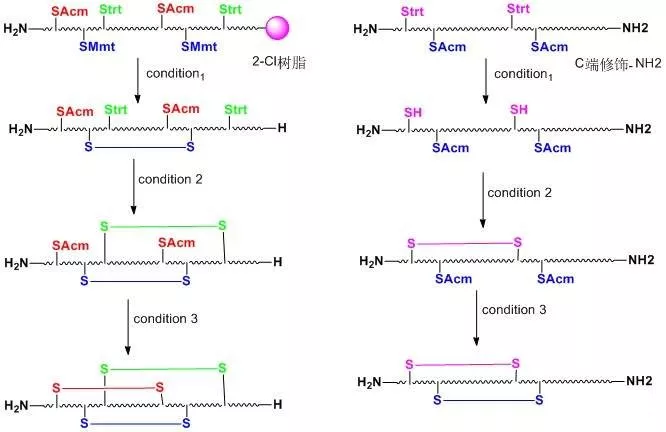
When two or more disulfide bonds need to be formed on a peptide chain, the reaction process becomes relatively complex.Before the solid-phase synthesis of polypeptides, it is necessary to design several pairs of disulfide bond formation order and method routes in advance, select different side-chain sulfhydryl protecting groups, and use their different properties to oxidize step by step to form two or more pairs of disulfide bonds.Commonly used thiol protecting groups include trt, Acm, Mmt, tBu, Bzl, Mob, Tmob and other groups.We list two routes of formation of multiple pairs of disulfide bonds on polypeptides synthesized using 2-Cl resin and Rink resin as carriers, respectively:
Description 2:
Three different groups of protecting groups were used to protect the thiol groups of Cys, namely Trt, STmp and Acm.